
Hi everyone, today I want to talk to you about the risks of acetone. Acetone is a common organic solvent that is widely used in many fields, such as cosmetics, furniture manufacturing, and printing. However, we should also be alert to the potential risks of acetone. First, acetone is volatile and it releases toxic gases. If exposed to high concentrations of acetone gas for a long time, it may pose a potential threat to our health. The volatility of acetone also means that proper measures need to be taken during storage and use, and good ventilation should be maintained to avoid exposure to high concentrations of acetone gas. Secondly, acetone is a flammable substance with a low flash point. It may burn as long as it comes into contact with open flames or high temperatures. Therefore, be sure to stay safe when using acetone. It is forbidden to store acetone near flammable materials and keep it away from open flames and high-temperature sources. In addition, long-term exposure to acetone may cause some health problems. Acetone irritates the skin and eyes, which may cause dryness, itching, or redness, and even cause eye pain and blurred vision. Therefore, after contact with acetone, wash your skin and eyes immediately and seek medical help. Finally, acetone also has certain ecological risks. If not properly handled, acetone may enter the soil and water, causing potential harm to the ecosystem. Therefore, when using acetone, be sure to pay attention to environmental protection and properly handle and dispose of waste. Hope that through the sharing, I can remind everyone to be vigilant about the risks of acetone.
Read More
Butyl Acrylate is a chemical compound with the formula C7H12O2 and is commonly known as butyl ester of acrylic acid. It is a colorless liquid with a fruity odor. Butyl acrylate has various applications in different industries due to its unique chemical properties. Here are some of the primary uses of butyl acrylate: 1. Coatings and Paints: Butyl acrylate is primarily used as a raw material in the production of water-based coatings and paints. It is a key component in acrylic emulsion polymers, which provide excellent adhesion, flexibility, and weather resistance. 2. Adhesives: Butyl acrylate is used in the manufacturing of pressure-sensitive adhesives (PSAs). These adhesives are commonly used in tapes, labels, and other adhesive applications that require quick and strong bonding. 3. Textile Finishing: Butyl acrylate is employed in the textile industry as a finishing agent for fabric. It enhances the fabric’s strength, wrinkle resistance, and dye receptivity. 4. Paper Coatings: Butyl acrylate is used in the production of paper coatings, which provide enhanced printability, smoothness, and water resistance to paper products like magazines, catalogs, and packaging materials. 5. Emulsion Polymers: Butyl acrylate is a key component for the production of emulsion polymers used in various applications such as carpet backing, non-woven textiles, and construction materials. 6. Plastic Additives: Butyl acrylate is utilized as an additive in plastic formulations to improve impact resistance, flexibility, and weatherability. It is commonly incorporated into PVC, acrylic, and other plastic products. 7. Personal Care Products: Butyl acrylate is used in the production of personal care products such as hair sprays, nail polishes, and cosmetics, where its film-forming properties and adhesion are beneficial. If you need solvents like Butanone, Toluene, Xylene, Isopropanol, Styrene, Cyclohexanone, Ethanol, Methanol, Ethyl Acetate, N-butyl Acetate, Butyl Acrylate, etc., mainly used in paints, coatings, spray paints, printing, leather, ink, and pharmaceutical intermediates. You can contact Batong Chemical Group.
Read More
Hi everyone, today I want to talk to you about the risks of acetone. Acetone is a common organic solvent that is widely used in many fields, such as cosmetics, furniture manufacturing, and printing. However, we should also be alert to the potential risks of acetone. First, acetone is volatile and it releases toxic gases. If exposed to high concentrations of acetone gas for a long time, it may pose a potential threat to our health. The volatility of acetone also means that proper measures need to be taken during storage and use, and good ventilation should be maintained to avoid exposure to high concentrations of acetone gas. Secondly, acetone is a flammable substance with a low flash point. It may burn as long as it comes into contact with open flames or high temperatures. Therefore, be sure to stay safe when using acetone. It is forbidden to store acetone near flammable materials and keep it away from open flames and high-temperature sources. In addition, long-term exposure to acetone may cause some health problems. Acetone irritates the skin and eyes, which may cause dryness, itching, or redness, and even cause eye pain and blurred vision. Therefore, after contact with acetone, wash your skin and eyes immediately and seek medical help. Finally, acetone also has certain ecological risks. If not properly handled, acetone may enter the soil and water, causing potential harm to the ecosystem. Therefore, when using acetone, be sure to pay attention to environmental protection and properly handle and dispose of waste. Hope that through the sharing, I can remind everyone to be vigilant about the risks of acetone.
Read More
Butyl Acrylate is a chemical compound with the formula C7H12O2 and is commonly known as butyl ester of acrylic acid. It is a colorless liquid with a fruity odor. Butyl acrylate has various applications in different industries due to its unique chemical properties. Here are some of the primary uses of butyl acrylate: 1. Coatings and Paints: Butyl acrylate is primarily used as a raw material in the production of water-based coatings and paints. It is a key component in acrylic emulsion polymers, which provide excellent adhesion, flexibility, and weather resistance. 2. Adhesives: Butyl acrylate is used in the manufacturing of pressure-sensitive adhesives (PSAs). These adhesives are commonly used in tapes, labels, and other adhesive applications that require quick and strong bonding. 3. Textile Finishing: Butyl acrylate is employed in the textile industry as a finishing agent for fabric. It enhances the fabric’s strength, wrinkle resistance, and dye receptivity. 4. Paper Coatings: Butyl acrylate is used in the production of paper coatings, which provide enhanced printability, smoothness, and water resistance to paper products like magazines, catalogs, and packaging materials. 5. Emulsion Polymers: Butyl acrylate is a key component for the production of emulsion polymers used in various applications such as carpet backing, non-woven textiles, and construction materials. 6. Plastic Additives: Butyl acrylate is utilized as an additive in plastic formulations to improve impact resistance, flexibility, and weatherability. It is commonly incorporated into PVC, acrylic, and other plastic products. 7. Personal Care Products: Butyl acrylate is used in the production of personal care products such as hair sprays, nail polishes, and cosmetics, where its film-forming properties and adhesion are beneficial. If you need solvents like Butanone, Toluene, Xylene, Isopropanol, Styrene, Cyclohexanone, Ethanol, Methanol, Ethyl Acetate, N-butyl Acetate, Butyl Acrylate, etc., mainly used in paints, coatings, spray paints, printing, leather, ink, and pharmaceutical intermediates. You can contact Batong Chemical Group.
Read More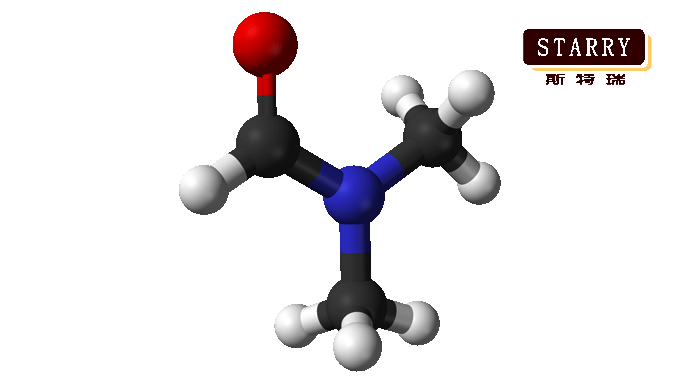
N, N-Dimethylformamide (DMF) is a versatile organic solvent with a wide range of applications in various industries. Here are some of the main uses of DMF: 1. Chemical Reactions: DMF is commonly used as a solvent in chemical reactions. It can dissolve a wide range of organic and inorganic compounds and is particularly useful for reactions involving polar and nonpolar compounds. 2. Pharmaceutical Industry: DMF is widely used as a solvent in the pharmaceutical industry for drug synthesis, formulation, and purification processes. It aids in dissolving and stabilizing various drug compounds. 3. Polymer Industry: DMF serves as a solvent and reaction medium in polymer synthesis, including the production of polyurethanes, polyamides, and polyacrylonitrile. It helps in dissolving and dispersing polymer precursors and facilitates the formation of homogeneous polymer solutions. 4. Electronics: DMF is used in the electronics industry as a solvent for cleaning, degreasing, and removing soldering residues from electronic components. It is also used in the production of electronic devices, such as semiconductors and printed circuit boards. 5. Textile Industry: DMF is employed as a solvent in the dyeing and finishing of textiles. It helps in dissolving and dispersing dyes, improving color uniformity and dye penetration into the fabric. 6. Adhesives and Sealants: DMF is used as a solvent in the formulation of adhesives and sealants. Its high solvency power allows for the dissolution of various adhesive components, enabling proper mixing and application. 7. Metal Processing: DMF is utilized in metal processing industries as a solvent for metal cleaning, degreasing, and electroplating processes. It helps in removing dirt, oils, and contaminants from metal surfaces. If you need solvents like Butanone, Toluene, Xylene, Isopropanol, Styrene, Cyclohexanone, Ethanol, Methanol, Ethyl Acetate, N-butyl Acetate, Butyl Acrylate, etc., mainly used in paints, coatings, spray paints, printing, leather, ink, and pharmaceutical intermediates. You can contact Batong Chemical Group
Read More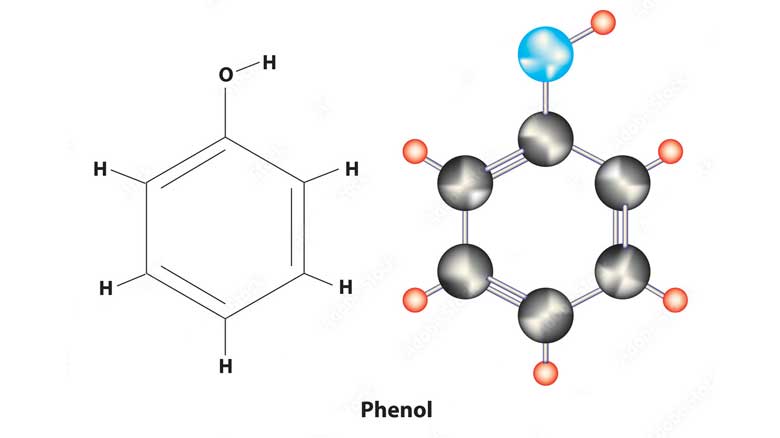
Phenol is a compound with antibacterial, anti-inflammatory, analgesic, and other effects. 1. phenol has strong antibacterial and anti-inflammatory effects, can kill various bacteria, fungi, and viruses, and effectively prevent and treat infectious diseases. 2. Phenol can relieve pain and discomfort by inhibiting pain transmission and reducing tissue inflammation and has a certain relief effect on mild pain symptoms such as headache, toothache, and joint pain. 3. Phenol has a certain antioxidant effect, which can prevent the damage of free radicals to body cells and delay the aging process. It should be noted that phenol is toxic at high concentrations, should be used in accordance with the dosage prescribed by the doctor or the instruction manual, and should not be abused at will. It should be used with caution for sensitive groups and pregnant women. It should be noted that phenol is toxic at high concentrations, should be used in accordance with the dosage prescribed by the doctor or the instruction manual, and should not be abused at will. It should be used with caution for sensitive groups and pregnant women.
Read More
Methyl Ethyl Ketone (MEK) and Acetone are both solvents used in various industries. They share some similarities but also have distinctive differences. Here are some of them: 1. Structure: The fundamental difference lies in their chemical structure. MEK consists of four carbon, eight hydrogen, and one oxygen atom, while Acetone consists of three carbon, six hydrogen, and one oxygen atom. 2. Strength: MEK is a stronger solvent than Acetone. It takes longer to evaporate and is often used for tasks that require a slower evaporation rate. 3. Application: Because of its stronger nature, MEK is often used in industrial applications such as in the manufacture of plastics, and textiles, and in the creation of paraffin wax. On the other hand, Acetone is commonly used as a nail polish remover in laboratories. 4. Safety: Both are flammable and have strong odors, but Acetone is generally considered safer and less damaging to the environment. 5. Cost: Acetone is usually cheaper than MEK. Remember, both substances should be used with care, as they can be harmful if mishandled. Always follow safety guidelines when using these chemicals.
Read More
The solvent is a liquid that can dissolve a solid, liquid, or gaseous solute and subsequently become a solution. The most common solvent in everyday life is water. The most common example is coffee or tea brewed in hot water. Solvents are usually clear, colorless liquids, and most of them have a distinctive odor. Solvents have the following main characteristics: 1. hydrophilicity: solvents and solutes can be broadly categorized as polar (hydrophilic) and non-polar (hydrophobic). 2. Boiling point: Another important property of solvents is the boiling point, which is related to the rate of evaporation. A small amount of a low-boiling solvent, such as ether, methylene chloride, or acetone, will evaporate in a few seconds at room temperature. High-boiling solvents, such as water or dimethyl sulfoxide, however, require higher. 3. Density: Most organic solvents are less dense than water, so they are lighter than water and are on top of water when layered. However, some organic solvents containing halogenated elements are usually more subdued than water and will sink to the bottom. This is of particular concern when using a dispensing funnel to dispense water and solvents.
Read More
Phenol, also known as carbolic acid, is an organic compound with the chemical formula C6H5OH. It is a white crystalline solid that has a sweet and acrid odor. Phenol is an important industrial chemical that is used as a starting material for the manufacture of many products, including plastics, pharmaceuticals, and dyes. Phenol is a weak acid and can undergo reactions typical of alcohols and carboxylic acids. It is soluble in water, alcohol, and ether, and has antiseptic properties, which makes it useful in medical applications. Phenol is also used as a disinfectant and in the production of resins, adhesives, and paints. Phenol can be toxic and harmful if inhaled, ingested, or in contact with the skin. It can cause burns and severe irritation to the skin, eyes, and respiratory tract, and can be fatal if ingested in large amounts. Therefore, it should be handled with care and proper personal protective equipment should be worn when working with phenol. Phenol has a variety of functions in industry and other fields. Some of the functions of phenol are: 1. Production of Plastics and Resins: Phenol is a primary raw material for the production of various plastics, including Bakelite and phenolic resins. These are used in the manufacturing of electrical equipment, automotive parts, and household goods. 2. Manufacture of Pharmaceuticals: Phenol is used to produce a variety of analgesics, antiseptics, and other pharmaceuticals. It is also used to synthesize salicylic acid, which is the active ingredient in aspirin. 3. Disinfectant: Phenol has antiseptic properties and is used as a disinfectant for various surfaces and medical equipment. It is also used in mouthwashes and throat sprays to kill bacteria. 4. Extraction of Essential Oils: Phenol is used to extract essential oils from plants and herbs. It is also used as a preservative in these oils to extend their shelf life. 5. Laboratory Research: Phenol is used in various laboratory procedures as a reagent, solvent, and fixative.
Read More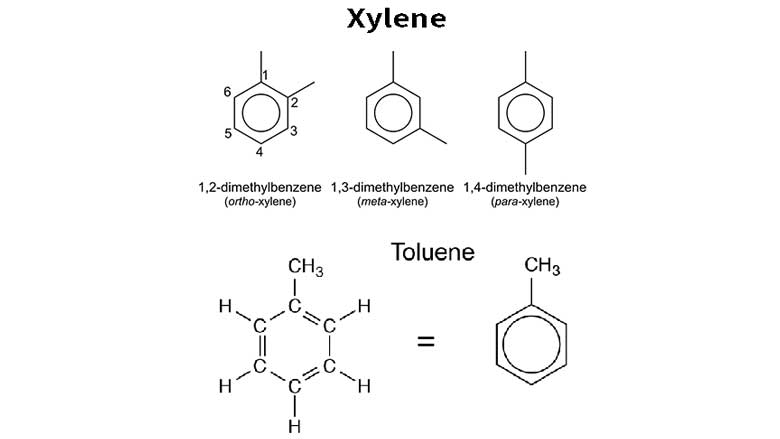
Toluene and xylene are both aromatic hydrocarbons that are commonly used as solvents and are produced from crude oil. While they share some similarities, there are also differences in their functions and properties: 1. Chemical Structure: Toluene (C7H8) has a benzene ring with a methyl group attached, while xylene (C8H10) has a benzene ring with two methyl groups attached at different positions. 2. Solvent Properties: Both toluene and xylene are effective solvents for a wide range of materials, including paints, lacquers, adhesives, and coatings. However, xylene is often considered a more powerful solvent compared to toluene. Xylene is commonly used in applications that require stronger solvency, such as in the printing, rubber, and leather industries. 3. Applications: Toluene is widely used in the production of gasoline additives, explosives, synthetic fibers, and pharmaceuticals. It is also used as a solvent in paints, coatings, and adhesives. Xylene, on the other hand, is commonly used in the production of polyester fibers, dyes, and coatings. It is also used as a cleaning agent in the electronics industry and as a solvent in the manufacture of rubber, leather, and printing inks. 4. Isomer Composition: Xylene exists in three isomeric forms: ortho-xylene, meta-xylene, and para-xylene. These isomers have slightly different chemical properties and are used in different applications. Toluene does not have isomers. 5. Toxicity: Both toluene and xylene are toxic and can cause health problems.
Read More