Home > News
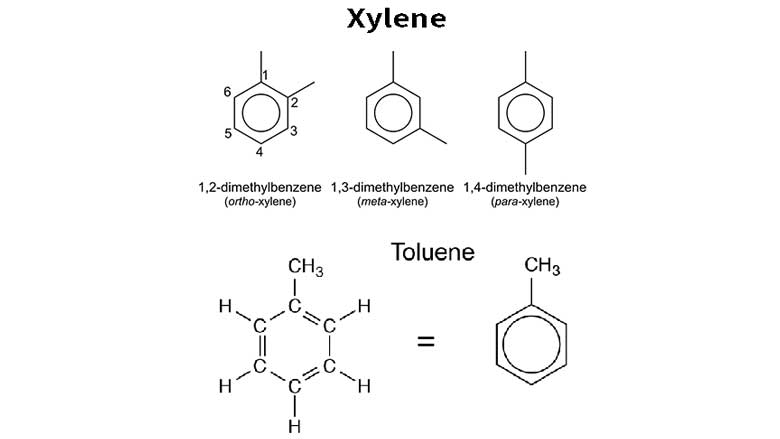
Toluene and xylene are both aromatic hydrocarbons that are commonly used as solvents and are produced from crude oil. While they share some similarities, there are also differences in their functions and properties:
The domestic market price of methyl ethyl ketone (MEK) is showing a trend of regional convergence, with current ...
Read More
Ethyl acetate in East China, affected by plant maintenance, some enterprises have slightly raised prices. In Sou...
Read More
The domestic acetone market has shown a clear downward trend in prices. The global acetone market is influenced ...
Read More
As of August 15, 2025, the aromatics product market exhibits characteristics of price differentiation and supply...
Read MoreLeave your message and we will get in touch with you as soon as possible.